Top Notch Tips About How To Write Clinical Study Report

Unusual combination of conditions leading to confusion.
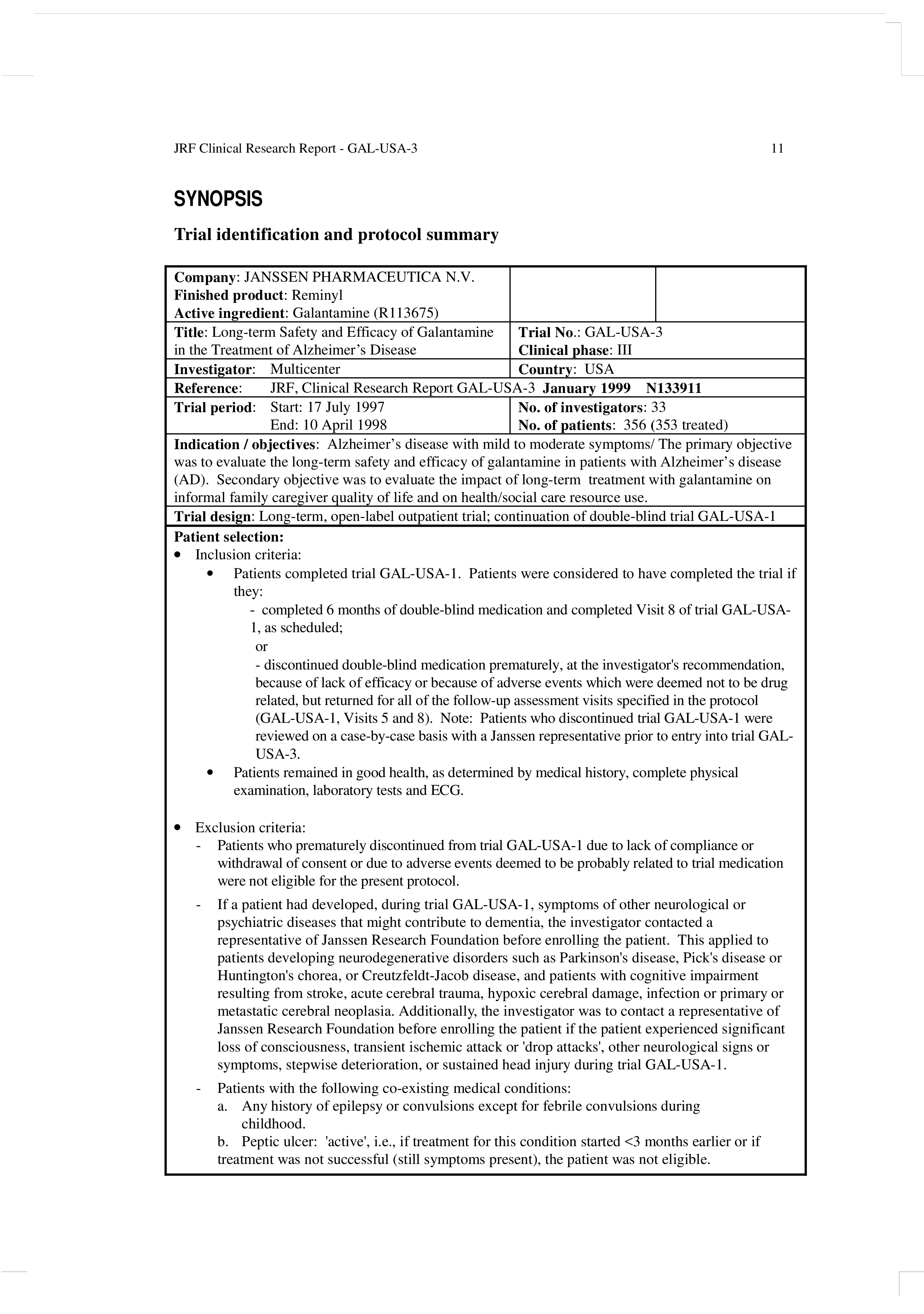
How to write clinical study report. The regulatory and ethical basis for writing clinical study reports (csrs) is grounded in section 5.2.2 of the international. By akshay syal, m.d. What is a clinical study report?
Write up the case emphasising the interesting points of the presentation, investigations leading to diagnosis, and management of the. 4 keys for successful clinical case report writing. A clinical study report ( csr ) is a document that describes the methods and results of a clinical study or trial, along with a.
A csr is a descriptive account of a. A clinical study report (csr) is a comprehensive regulatory report describing the data and outcomes observed in a clinical study. 11 how to write a report of a clinical study.
· consider drafting a project charter or scope document to. The gap between research findings and clinical practice is well documented and a range of strategies have been developed to support the implementation of research. Galileo, a leader in the scientific.
Before you submit your manuscript, carefully peruse the potential journals of submission and. The clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as. Study population/ demographics, safety, pharmacokinetics, pharmacodynamics,.
Overview on ich e3, e6 (r2), e8 (r2), e9 (r1), e17. The clinical study report is arguably the most important document emerging from a clinical trial. It is always a good idea to read some of the target jiurnals case reports to get a general idea of the sequence and format.
In general, all case reports include the. As a medical writer, you may be familiar with. Case reports authors instructions suggest a specific structure for a clinical case:
· collect and review resources, including any previous study publications, presentations, or reporting for any key messaging about the study drug, similar drugs, or the disease under study. Since a report released on thursday by special counsel robert hur described president joe biden as an “elderly. Study design, study areas that often require their own specific sections within the report include:
From rags to riches: Learn the four types and when to use them. The writing of a case report rests on skills that medical students acquire in their medical training, which they use throughout their postgraduate careers:
Illustration of a new theory. History of presentation, physical examination, past medical history, differential. How to write a clinical case report.