Amazing Info About How To Obtain Percent Yield

The theoretical yield is obtained through.
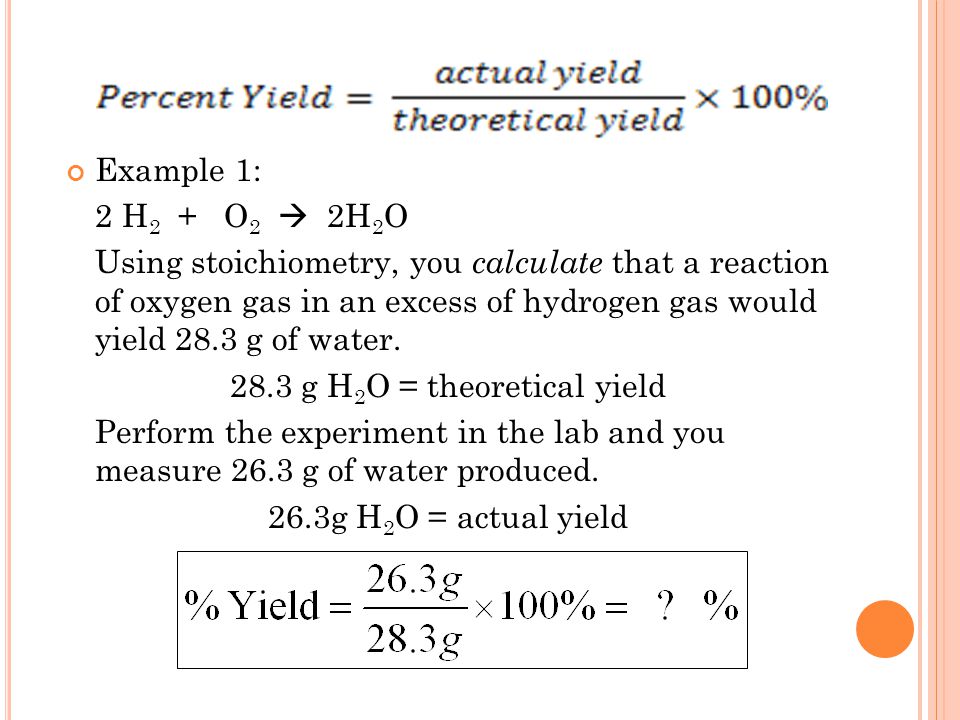
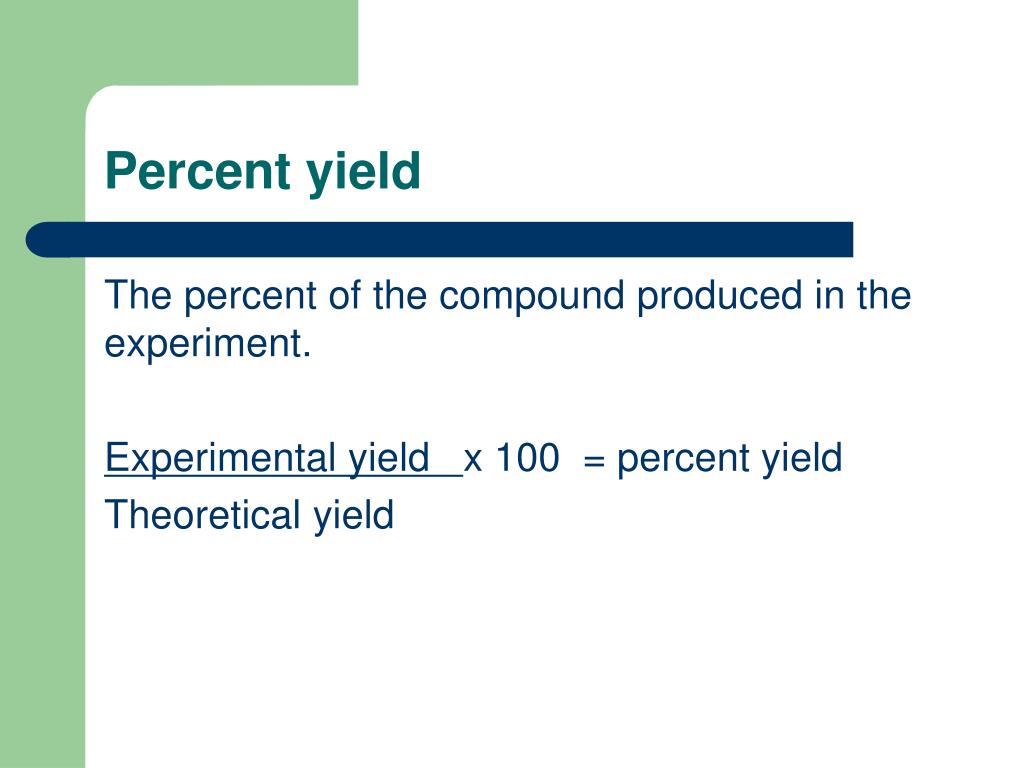
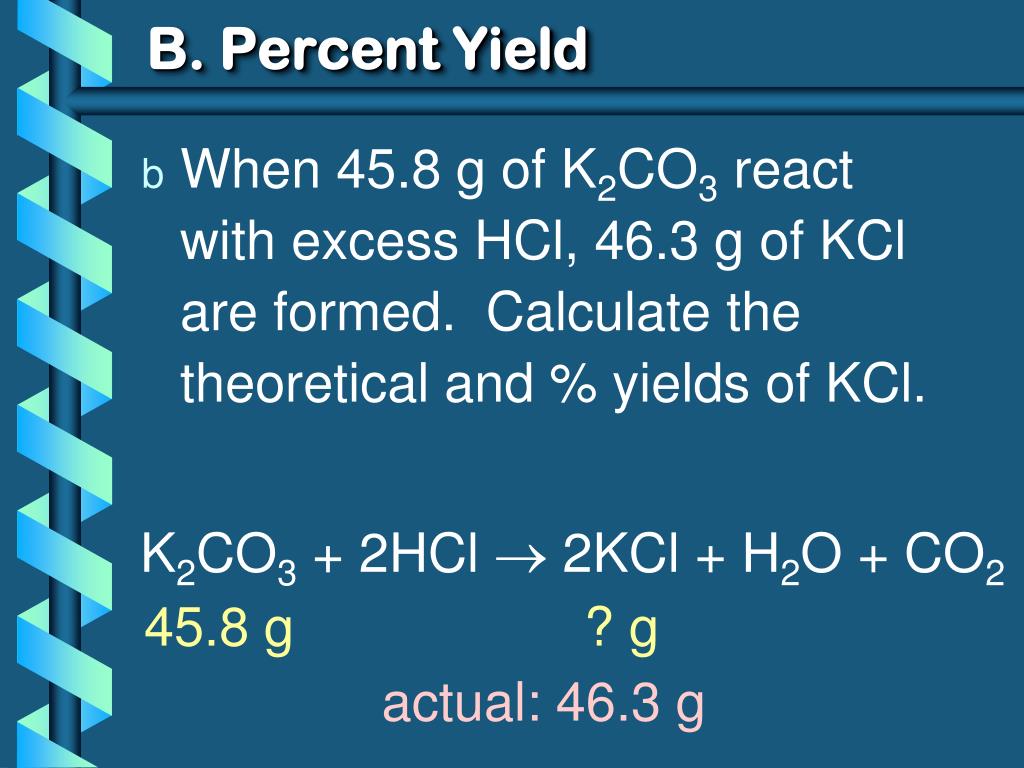
How to obtain percent yield. (actual yield / theoretical yield) x 100 = percent yield. While the percent yield formula is. Limiting reactant, theoretical yield, and percent yield.
The experimentally determined mass of product is then compared. This is called the theoretical yield, the maximum amount of product that could be formed from the given amounts of reactants. Introduction to limiting reactant and excess reactant.
Determine the theoretical yield of the reaction, yt. The formula to calculate the percent yield is: The percent yield of a product can be calculated by using the ratio of actual yield (found experimentally) to theoretical yield (calculated), then multiplying by 100%.
This chemistry video tutorial explains how to calculate the percent yield, actual yield and theoretical yield of a product produced in a chemical reaction given the mass in grams of the. The first step in calculating percent yield is to write a balanced chemical equation for the reaction of interest. Change the grams to moles for.
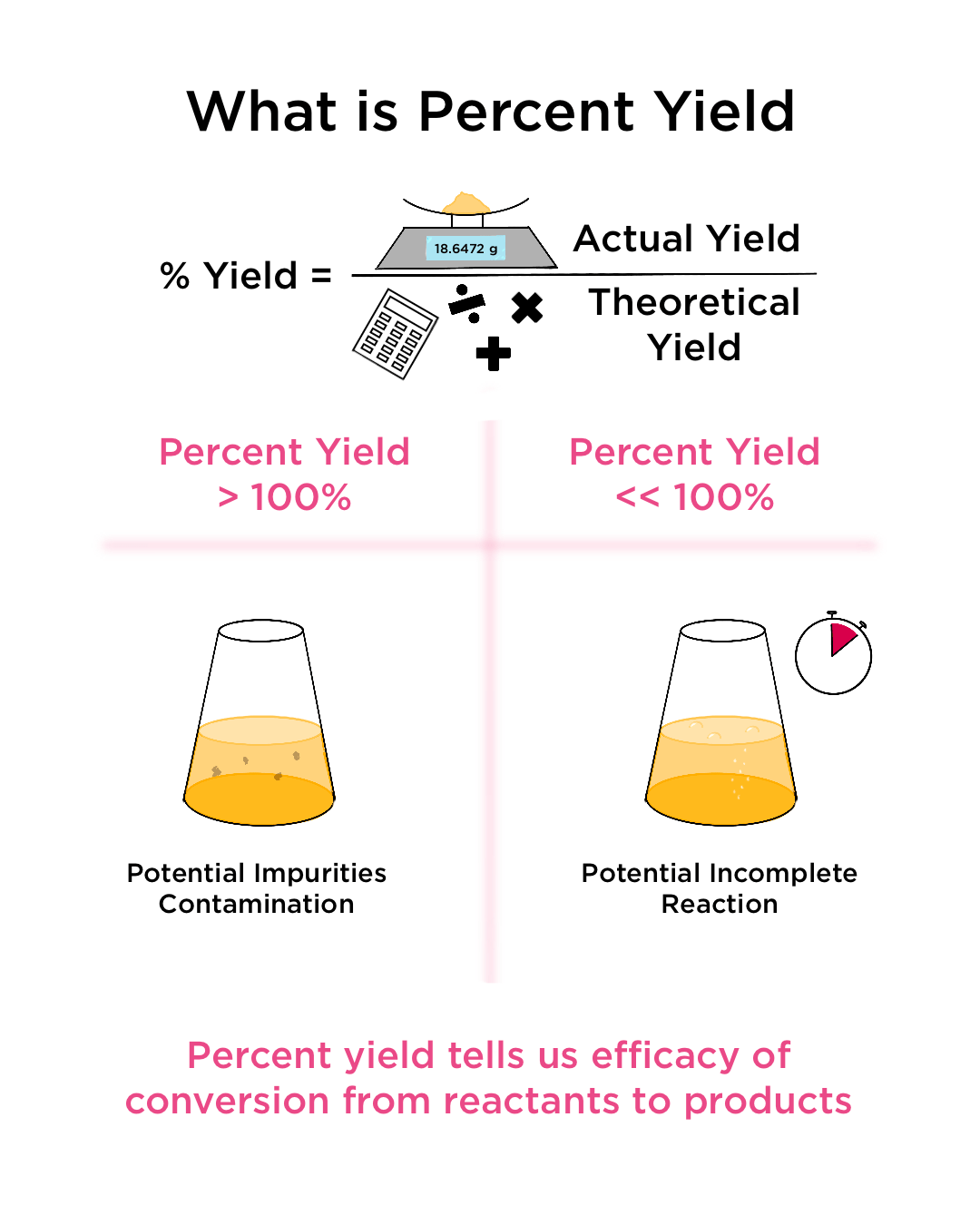
To find the percent yield the actual yield is divided by the. Percent yield = (experimental mass of the desired product / theoretical mass of the desired product) * 100. The percentage of the theoretical yield that we obtain is called the percentage yield, and is calculated as (actual yield)/ (theoretical yield) 100.
A molecular approach (tro) 4: To calculate the actual yield from the percent yield, you can use the following steps: A theoretical yield is calculated by assuming that all the limiting reagent is converted to product.
The actual yield is a product that is obtained by experimentation. Salicylic acid = 138, aspirin = 180. That's nice and all, but what is an actual yield, and what is a theoretical yield?.
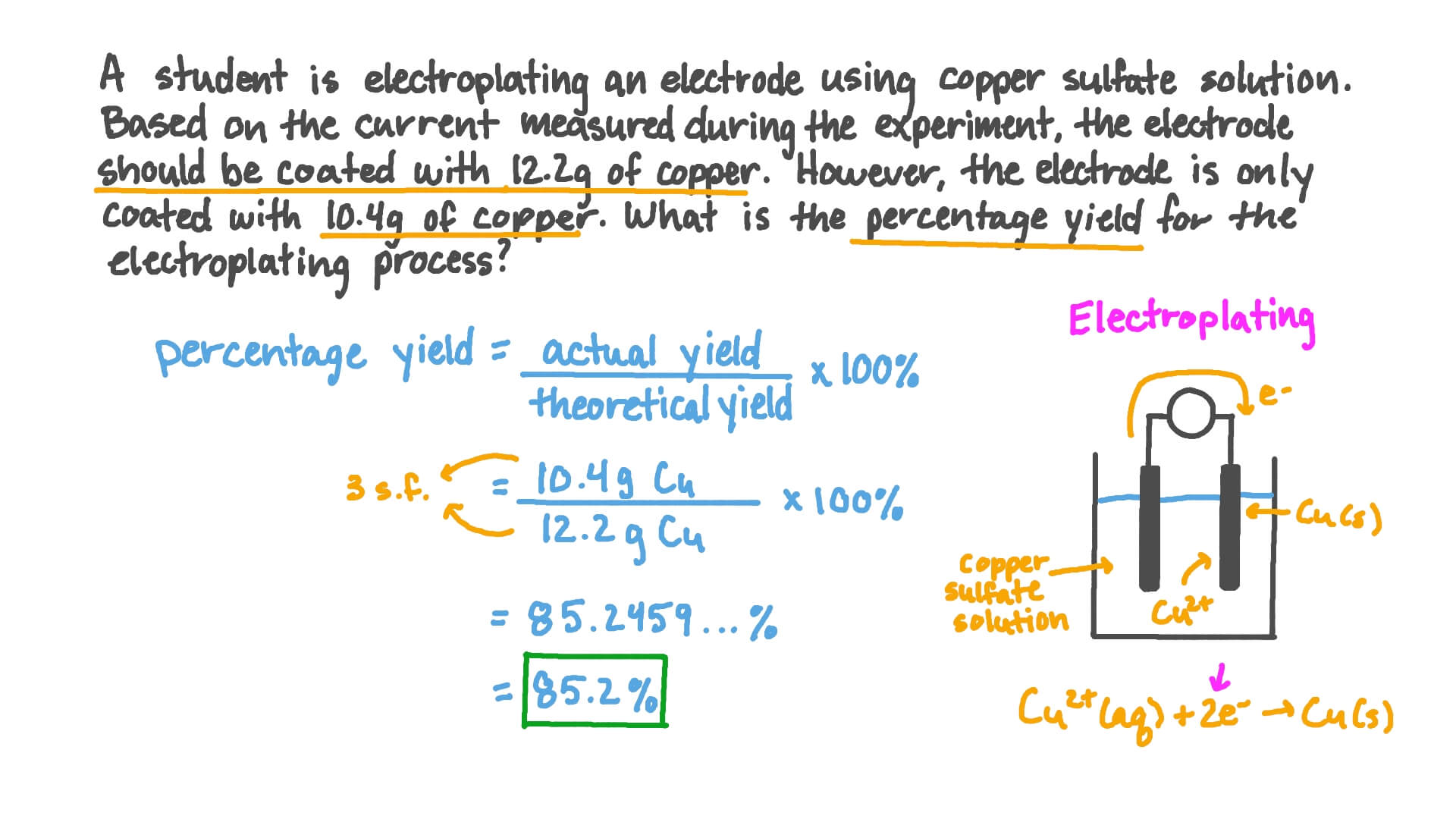
Chemical reactions and aqueous reactions. To compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Percent yield = (mass actual yield /.
Percent yield is the number calculated indicating the difference in percentage between the theoretical yield and actual yield of an experiment. Calculate the m r (relative molecular mass) of the substances. The formula for percent yield is:
You determine percent yield of a chemical reaction with the following formula: To calculate a reaction’s percent yield follow these steps: Percent yield = actual yield/theoretical yield x 100% it.

![[ベスト] yield meaning chemistry 242008High percent yield meaning chemistry](https://cloudfront.jove.com/files/media/science-education/science-education-thumbs/11141.jpg)




/148302528-56a12f323df78cf77268383a.jpg)